How Limestone is Formed, Where Does it Form?
Limestone, a sedimentary rock composed primarily of calcium carbonate (CaCO₃), forms via two predominant pathways: biogenic precipitation and abiogenic precipitation. Understanding these processes necessitates an examination of the relevant chemistry, geological forces, and temporal scales involved.
Calcium carbonate (CaCO₃), present in two primary crystalline forms – calcite and aragonite – serves as the fundamental building block of limestone. This ubiquitous mineral, readily dissolved in water, particularly seawater, becomes the critical reactant in the formation process.
Limestone serves as a valuable geological archive, preserving fossils and recording environmental conditions of its formation.
Limestone is a sedimentary rock that forms from the accumulation of the remains of marine organisms, such as coral and shells, and the precipitation of calcium carbonate from water. Two distinct pathways contribute to limestone formation:
 |
| How Limestone is Formed. |
Biogenic Precipitation
Biogenic precipitation limestone formed primarily from the remains of organisms that secrete calcium carbonate, such as shells, skeletons, and exoskeletons.
The foundation of biogenic limestone lies in the remarkable ability of marine organisms to utilize calcium carbonate from the surrounding water to build their shells and skeletons. This diverse cast includes:
- Corals: Reef-building corals, with their intricate calcium carbonate skeletons, are crucial contributors in tropical and subtropical settings.
- Mollusks: Clams, oysters, and other shelled creatures add significantly to the carbonate sediment.
- Foraminifera: Microscopic, single-celled organisms with calcareous tests contribute vast amounts to deep-sea sediments.
- Algae: Certain types of algae also play a role, particularly in freshwater environments.
As these organisms grow, reproduce, and eventually die, their calcium carbonate-rich remains rain down onto the seabed, accumulating in layers over millennia. These organic-rich sediments, called oozes, form the initial stage of biogenic limestone.
Compaction: Over vast timescales, the weight of accumulating sediments compacts these organic-rich layers, expelling water and air. This compaction, akin to consolidating loose sand, paves the way for the subsequent step: cementation. Minerals like calcite, dissolved in percolating water, act as a natural adhesive, binding the calcium carbonate particles together, transforming them into a hardened mass – the nascent stages of limestone.
Cementation: Minerals like calcite, dissolved in water percolating through the sediment, act as a natural glue. These minerals bind the calcium carbonate particles together, solidifying the rock.
Uplift and exposure: Eventually, geological forces can uplift the seafloor, bringing the once-buried limestone to the surface. This exposed limestone becomes visible as rock formations and can be studied by geologists to understand the past environment and the organisms that lived there.
The final characteristics of biogenic precipitation limestone can vary depending on the types of organisms that contributed to its formation, the size and shape of the organic fragments, and the environmental conditions during its deposition. Some limestones are fine-grained and dense, while others are coarse-grained and porous. The color can also range from white to gray, brown, or even black, depending on the presence of impurities like iron or organic matter.
Chemical Precipitation
Chemical precipitation representing the primary non-biological process. This mechanism involves the direct conversion of dissolved calcium carbonate (CaCO₃) into solid limestone without the involvement of living organisms.
Environmental Triggers: Changes in temperature, pressure, or salinity within a body of water can disrupt the equilibrium of dissolved calcium carbonate. This disruption can lead to its precipitation as solid crystals, which subsequently accumulate on the seabed.
Dissolution: The initial step involves the dissolution of minerals containing calcium and carbonate ions in water. This can occur through weathering of rocks on land or from interactions with marine sediments.
Supersaturation: When the water becomes saturated with dissolved calcium and carbonate ions, it reaches a state of supersaturation. This means the water holds more dissolved ions than it can normally hold under the prevailing conditions.
Precipitation: Under the influence of the aforementioned factors, dissolved calcium and carbonate ions combine to form solid CaCO₃ crystals. These crystals precipitate out of solution, accumulating on the seabed or other surfaces.
Compaction and Cementation: Over time, the accumulated crystals undergo compaction due to the weight of overlying sediments. Additionally, dissolved minerals can act as cements, binding the crystals together and forming solid limestone.
Unique Insights: While lacking fossils, the composition and layering of abiogenic limestones provide insights into paleoclimate, water chemistry, and geological events.
Distinguishing Features of Chemical Precipitation Limestone
Limestone formed through chemical precipitation often possesses distinct characteristics:
Absence of Fossils: Unlike biogenic limestone, this type typically lacks recognizable fossils as it doesn't rely on organism remains.
Crystal Fabric: Precipitated crystals often exhibit characteristic shapes and arrangements, providing clues about the specific precipitation mechanism.
Types of Chemically Precipitated Limestones
Oolitic limestone: Characterized by small, rounded grains (oolites) formed through precipitation around sand particles in agitated shallow waters.
Travertine: Deposited from hot springs or rivers rich in dissolved calcium carbonate, often forming layered structures like terraces or waterfalls.
Tufa: Similar to travertine but formed in freshwater lakes and springs.
Beachrock: Formed in the intertidal zone through rapid precipitation of calcium carbonate due to evaporation, sunlight, and wave action.
 |
| Oolitic limestone |
Variations in Limestone Composition and Texture
There are different types of limestone, and variations in the formation process can lead to differences in the rock's characteristics.
The relative contribution of biogenic and chemical precipitation varies depending on environmental conditions and geological history.
Other minerals, such as silica and clay, can be incorporated during sedimentation, influencing the final rock properties.
The intensity of compaction and cementation determines the density, porosity, and texture of the resulting limestone.
The formation process, the presence of additional minerals, and the intensity of compaction and cementation contribute to the diverse range of limestone types observed. From the fine-grained and crumbly chalk to the dense and crystalline marble, each variety reflects its unique geological journey.
 |
| limestone with fossil |
Conclusion
The formation of limestone involves complex interplay between biological and chemical processes occurring over vast timescales. Understanding these mechanisms sheds light on Earth's dynamic history and the formation of valuable geological resources.
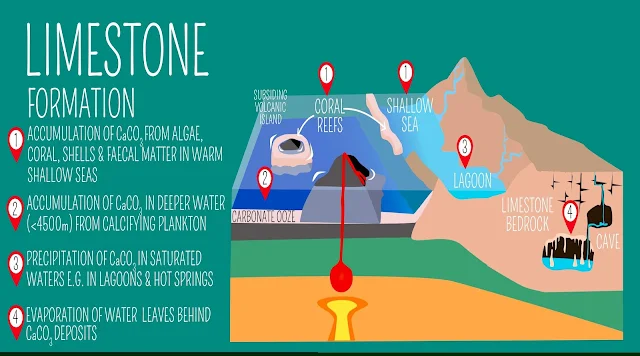
Where Does Limestone Form?
Limestone can form in various environments, primarily driven by the factors influencing calcium carbonate precipitation and accumulation. Here's a breakdown of the key locations:
Marine Environments
Shallow, Warm Seas: The most common setting for limestone formation is shallow, warm marine environments, such as continental shelves and platforms. These areas favor abundant calcifying organisms (corals, mollusks, foraminifera) that contribute their shells and skeletons, leading to biogenic limestone formation.
Tropical Lagoons and Reefs: These environments support high biological productivity, resulting in significant organic sediment accumulation rich in calcium carbonate. Additionally, chemical precipitation of calcium carbonate can occur due to factors like evaporation and increased sunlight penetration.
Freshwater Environments
Lakes and Rivers: While less common than marine settings, freshwater bodies can also host limestone formation through chemical precipitation or biological activity of freshwater calcifying organisms (e.g., freshwater snails, ostracods). The resulting limestone tends to be less abundant and more localized compared to marine deposits.
Terrestrial Environments
Evaporative Environments: In arid regions with high evaporation rates, calcium carbonate can precipitate from groundwater or surface water, forming deposits like caliche and travertine. This process is mainly chemical, but microbial activity can also play a role.
Cave Systems: Within caves, dissolution of limestone by acidic water leads to the formation of speleothems like stalactites and stalagmites. These features arise from re-precipitation of the dissolved calcium carbonate under altered chemical conditions within the cave system.
Hot Springs: In some cases, limestone can form in hot springs where calcium carbonate precipitates from the water due to changes in temperature and pressure. This process often results in the deposition of travertine, a type of limestone.
 |
| Limstone formed by Chemical Precipitation |
Factors Influencing Location
Several factors influence where limestone forms:
Presence of Calcium Carbonate Source: Readily available dissolved calcium carbonate, either from seawater, freshwater, or weathering of carbonate rocks, is essential for limestone formation.
Suitable Environmental Conditions: Warm, shallow marine environments favor the growth of calcifying organisms, while arid conditions support chemical precipitation in terrestrial settings.
Time and Stability: Limestone formation is a slow process requiring millions of years of accumulation and preservation. Stable geological conditions are crucial for this process to occur efficiently.
By understanding these factors and different environments, we can better predict where limestone deposits might be found and appreciate the diverse geological settings that contribute to this valuable rock.
Conclusion
The scientific discourse surrounding limestone elucidates the intricacies of its formation, rooted in organic accretion, compaction, cementation, and chemical precipitation. Recognizing the specific environments where limestone manifests underscores the geological significance of this sedimentary rock, enriching our comprehension of Earth's intricate processes.